How to Test the Quality of Fisetin Powder
2024-05-13 22:07:43
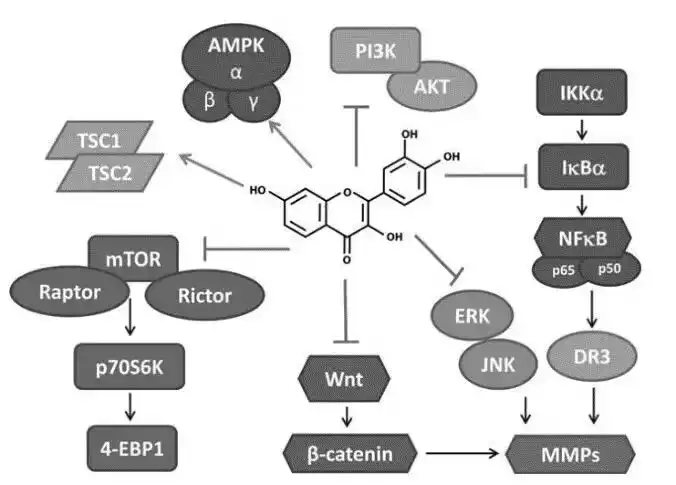
Fisetin, a flavonoid compound found in various fruits and vegetables, has gained attention for its potential health benefits. As a dietary supplement, it is important to ensure the quality and purity of fisetin powder.
We are fisetin supplier. We explore different methods for testing the quality of fisetin, focusing on its purity, identification, and quantification. We are the only enterprise using external standard method, the content of fisetin can be higher than 98%+.

Purity Testing:
To assess the purity of fisetin powder, several techniques can be employed:
a. High-Performance Liquid Chromatography (HPLC):
HPLC is a commonly used method to determine the purity of fisetin. It separates and quantifies individual components in a sample based on their retention times and UV absorbance. By comparing the peak area of fisetin to that of impurities or related compounds, the purity of fisetin can be determined.
b. Thin Layer Chromatography (TLC): TLC is a commonly used technique for the identification and preliminary assessment of fisetin powder. It involves separating the individual components of the sample on a thin layer of adsorbent material and comparing their migration patterns with reference standards.
Chemical Composition Analysis:
a. Fourier Transform Infrared Spectroscopy (FTIR): FTIR is a technique that analyzes the absorption and transmission of infrared light by the sample. It helps identify the functional groups present in fisetin and provides information about its chemical structure.
b. Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy provides detailed information about the molecular structure of fisetin. It can confirm the presence of specific protons and carbons, aiding in its identification and quality assessment.
Impurity Analysis:
a. Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is a powerful technique for analyzing the presence of impurities, such as pesticides or solvents, in fisetin powder. It involves separating and identifying the individual components of the sample based on their mass and molecular structure.
b. Heavy Metal Testing: Fisetin should undergo analysis for heavy metal content to ensure compliance with safety standards. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is commonly employed for accurate quantification of heavy metal levels.
Microbiological Analysis:
Fisetin should be tested for microbial contamination to ensure its safety for use. Microbiological analysis includes tests for total viable count, yeast, mold, and specific pathogenic bacteria. These tests are performed using standard microbiological methods.
Thorough testing of fisetin powder is crucial to ensure its quality and safety. Identification and purity testing, chemical composition analysis, impurity analysis, and microbiological analysis are key aspects of quality assessment.
Please send email to admin@chenlangbio.com if you want more information about fisetin.
Send Inquiry
Related Industry Knowledge
- Can Cetyl Tranexamate HCL Be Combined with Other Skincare Ingredients?
- Is Pyrrolidinyl Diaminopyrimidine Oxide Effective Against Hair Loss?
- Red Wine Extract Powder for Skin Health and Beauty
- What is the Function of Ectoine
- What Does Alpha-GPC Do to the Brain
- Is Tongkat Ali Extract Powder Eurycomanone Powder Safe
- when Should I Take Marigold Extract Powder Lutein Morning or Night
- Where to Buy Praziquantel Powder
- What are the Benefits of Turkesterone
- Are Lutein and Zeaxanthin the Same Thing









